|
|
|
|
|
挖洞小能手:蚂蚁为了在啃开木头做了哪些适应性改变 | BMC Journal |
|
|
论文标题:Skeletomuscular adaptations of head and legs of Melissotarsus ants for tunnelling through living wood
期刊:Frontiers in Zoology
作者:Adam Khalife, Roberto A. Keller, Johan Billen, Francisco Hita Garcia, Evan P. Economo and Christian Peeters
发表时间:2018/08/14
数字识别码:10.1186/s12983-018-0277-6
原文链接:https://frontiersinzoology.biomedcentral.com/articles/10.1186/s12983-018-0277-6?utm_source=wechat&utm_medium=social&utm_content=organic&utm_campaign=BSCN_1_CelZH_WeChat_DailyPost
微信链接:https://mp.weixin.qq.com/s/L0oYos0ZmqULt83twCob7w
Melissotarsus蚁与蚧壳虫之间存在一种奇妙的共生关系,这种关系使得这些蚂蚁发生了特殊的适应性改变,改变后的蚂蚁能够在立木中通过啃咬挖掘出隧道。Christian Peeters博士在Frontiers in Zoology上新发表的一篇文章带我们领略了这些蚂蚁非凡的形态学特化。

上图描绘了正在啃咬中的工蚁,它在挖隧道时用特殊的腿靠墙来支撑自己的身体。
虽然有数千种蚂蚁以树为家,但通常它们都在已存在的洞中筑巢,这些洞可能是天然形成的,或由甲虫幼虫挖掘而成。只有少数几种蚂蚁能够咬碎干木,或者在树以外的地方筑巢。而Melissotarsus工蚁则以极其巧妙的方式在树木中通过啃咬开辟出了条条隧道,这也使得他们与盾蚧科蚧壳虫(Diaspididae)建立起了一种共生关系。这些长约2毫米的小蚂蚁具有增大的头部,它们的头部被大量肌肉包裹,用于在啃咬时控制上颚的开合。表皮内突能够帮助固定从各个角度延伸的肌肉纤维,使它们填满头部可用空间。

树干中颇为壮观的隧道网
对于昆虫来说,打开上颚几乎不需要什么力量,因此控制上颚打开和闭合的肌肉存在明显的不对称性。然而在Melissotarsus蚁中,打开上颚的肌肉却格外强壮,这是因为工蚁在向前挖掘时,需要推开被啃碎的木头。此外,Melissotarsus蚁的上颚形状也发生了一些变化,从而可以利用杠杆原理进一步放大肌肉的力量。同时,它们上颚末端的几丁质基质里还结合有纳米级锌簇,这进一步增强了它们的挖掘能力。
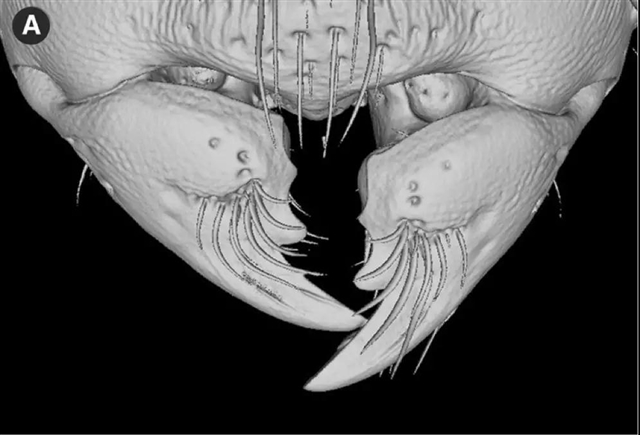
Melissotarsus工蚁的砍木上颚
这个强有力的啃咬能力还与四条后腿特化和充有肌肉的基节(髋关节)增大有关,特化后的后腿能够在挖掘时撑住隧道的侧壁。这些肢体方面的适应性变化非常极端,导致工蚁出了隧道就无法行走。这也基本证实这些工蚁需要完全依赖于隧道里的盾蚧科蚧壳虫来获取食物。除此之外,它们的前腿长满了毛发,用于将头部腺体产生的丝抽离出来。这些丝和木屑用于修复隧道中的损坏部分。在进化过程中,Melissotarsus蚁失去了它们的螫针,因此它们只能用这种丝质的结构进行防御,尤其是防御其他的树栖蚂蚁入侵挖好的隧道抢夺虫卵。一旦我们用刀划开Melissotarsus蚁挖的隧道,它们就会立刻开始修补,因此我们没有办法直接观察它们啃咬木头等其他行为。

Melissotarsus工蚁和与其共生的盾蚧科蚧壳虫
功能性形态学
大多数种类的蚂蚁都生活在木头或泥土的隧道内,但是Melissotarsus工蚁的适应性变化格外新奇。我们推断腿部的变化是为了在挖掘过程中起到支撑的作用。值得一提的是,Melissotarsus蚁后形态完全正常,拥有比较典型的翅膀和腿以及较大的眼睛。与之形成对比的是,工蚁们由于一生都在树木中挖洞,从而导致它们的眼睛发生了高度退化(这一现象在树栖蚂蚁中还未知),大脑(特别是视叶)也极度简化,为更发达的上颚肌肉腾出了空间。这种形态上的特化只在社会性昆虫中才可能出现,因为蚁后在建立蚁群初期需要能够飞往各处并在宿主树木之外进行活动。
摘要:
Background
While thousands of ant species are arboreal, very few are able to chew and tunnel through living wood. Ants of the genus Melissotarsus (subfamily Myrmicinae) inhabit tunnel systems excavated under the bark of living trees, where they keep large numbers of symbiotic armoured scale insects (family Diaspididae). Construction of these tunnels by chewing through healthy wood requires tremendous power, but the adaptations that give Melissotarsus these abilities are unclear. Here, we investigate the morphology of the musculoskeletal system of Melissotarsus using histology, scanning electron microscopy, X-ray spectrometry, X-ray microcomputed tomography (micro-CT), and 3D modelling.
Results
Both the head and legs of Melissotarsus workers contain novel skeletomuscular adaptations to increase their ability to tunnel through living wood. The head is greatly enlarged dorsoventrally, with large mandibular closer muscles occupying most of the dorsal half of the head cavity, while ventrally-located opener muscles are also exceptionally large. This differs from the strong closing: opening asymmetry typical of most mandibulated animals, where closing the mandibles requires more force than opening. Furthermore, the mandibles are short and cone-shaped with a wide articulatory base that concentrates the force generated by the muscles towards the tips. The increased distance between the axis of mandibular rotation and the points of muscle insertion provides a mechanical advantage that amplifies the force from the closer and opener muscles. We suggest that the uncommonly strong opening action is required to move away crushed plant tissues during tunnelling and allow a steady forward motion. X-ray spectrometry showed that the tip of the mandibles is reinforced with zinc. Workers in this genus have aberrant legs, including mid- and hindlegs with hypertrophied coxae and stout basitarsi equipped with peg-like setae, and midleg femura pointed upward and close to the body. This unusual design famously prevents them from standing and walking on a normal two-dimensional surface. We reinterpret these unique traits as modifications to brace the body during tunnelling rather than locomotion per se.
Conclusions
Melissotarsus represents an extraordinary case study of how the adaptation to – and indeed engineering of – a novel ecological niche can lead to the evolutionary redesign of core biomechanical systems.
阅读论文全文请访问:
https://frontiersinzoology.biomedcentral.com/articles/10.1186/s12983-018-0277-6?utm_source=wechat&utm_medium=social&utm_content=organic&utm_campaign=BSCN_1_CelZH_WeChat_DailyPost
期刊介绍:
Frontiers in Zoology (https://frontiersinzoology.biomedcentral.com/, 3.627 - 2-year Impact Factor, 3.782 - 5-year Impact Factor) is an open access, peer-reviewed online journal publishing high quality research articles and reviews on all aspects of animal life.
(来源:科学网)
特别声明:本文转载仅仅是出于传播信息的需要,并不意味着代表本网站观点或证实其内容的真实性;如其他媒体、网站或个人从本网站转载使用,须保留本网站注明的“来源”,并自负版权等法律责任;作者如果不希望被转载或者联系转载稿费等事宜,请与我们接洽。