
可充电锌-空气离子电池具有安全、零污染、高能量、大功率、低成本及材料可再生等优点,其中空气电极中的氧气电催化剂是技术的关键。
近日,深圳大学赵伟研究员课题组报道了一种利用原子-界面调控的方法,合成了氮/硫掺杂的多孔碳担载的铁/钴单原子协同双催化剂,用作空气电极材料实现了优异的锌-空气电池循环充放电性能。通过多种实验表征技术并结合理论计算,确定了非对称配位的Fe-(N2S)和Co-(N2S)结构提高了电荷转移效率,降低了反应能垒,提升了ORR和OER反应性能。
2023年1月30日,该研究成果以“Simultaneously Engineering the Synergistic-Effects and Coordination-Environment of Dual-Single-Atomic Iron/Cobalt-sites as a Bifunctional Oxygen Electrocatalyst for Rechargeable Zinc-Air Batteries”为题,在线发表于ACS Catalysis期刊。
深圳大学高等研究院的赵伟研究员是该论文的唯一通讯作者,第一作者为深圳大学高等研究院博士后Ghulam Yasin。
研究人员成功制备了氮/硫掺杂的多孔碳担载的Fe/Co双位点单原子催化剂Fe,Co/DSA-NSC,用SEM、HRTEM、XRD、HAADF-STEM、XAS等对催化剂材料做了表征,文章里有详细介绍,这里不多做赘述,主要证明了催化剂的Fe/Co单原子双催化中心,以及金属Fe/Co单原子位点周围的化学配位结构与环境(Fig. 1)。
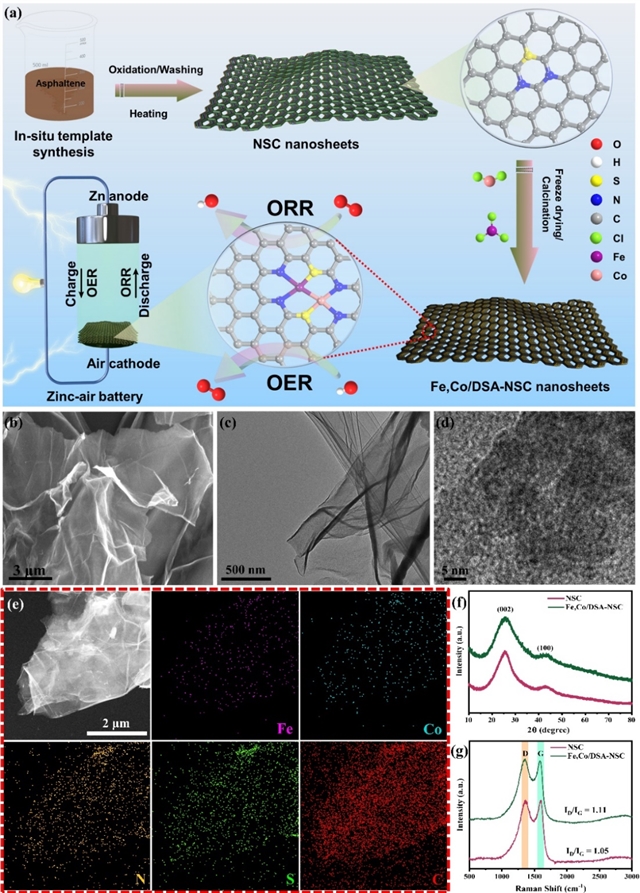
Fig. 1. (a) Schematic-sketch of the fabrication of the atomically-dispersed dual-metal active-sites in nitrogen/sulfur doped carbon-nanosheets. SEM-image (b) TEM image (c) and the HRTEM image (d) of the Fe,Co/DSA-NSC catalyst. (e) HAADF-STEM image of the Fe,Co/DSA-NSC and the subsequent elemental mappings of Fe, Co, N, S and C components. (f) XRD pattern (g) and the Raman spectra of NSC and Fe,Co/DSA-NSC catalysts.
催化剂Fe,Co/DSA-NSC的电化学测试情况:ORR的半波电位为879 mV,OER的过电位为210 mV(j=10 mA/cm2);ORR半电池测试,经过10000圈CV循环以后,半波电位只下降了6 mV;OER稳定性测试,在电流密度为10 mA/cm2的情况下经过20小时的连续运行以后,过电位只增加了0.1%。随后研究组尝试了应用,使用Fe,Co/DSA-NSC催化剂作为空气电极制成了可充电锌-空气电池,发现具有良好的充放电性能以及优异的循环稳定性:开路电压VOC为1.52 V,功率密度峰值为240 mW/cm2,好于常用的参考基准商业化Pt/C & IrO2作为空气电极的锌-空气电池(1.41 V&189 mW/cm2),在电流密度为10 mA/cm2情况下的比电容(specific-capacity)为748 mA h gZn−1,也好于Pt/C & IrO2的锌-空气电池(621 mA h gZn−1)。为了深入理解该催化剂优异性能的内在原因,研究组做了DFT理论计算,研究了催化剂材料的电子结构与OER/ORR反应机理,确定了非对称配位的Fe-(N2S)和Co-(N2S)结构是活性位点,并且Fe-(N2S)对于ORR具有更高的活性,而Co-(N2S)对于OER具有更高的活性。Fe/Co双催化活性位点大幅降低了ORR和OER反应能垒,提高了电荷转移效率,从而提升了ORR和OER性能,以及锌-空气电池与之对应的充放电性能,详细介绍请参见文章(Fig. 2、Fig. 3)。

Fig. 2. ORR linear-scan voltammogram (LSV) curves of NSC, Fe/SA-NSC, Co/SA-NSC, Fe,Co/DSA-NSC and Pt/C catalysts (a) and the consequential ORR-half-wave potentials (b). (c) LSV-curves of the Fe,Co/DSA-NSC sample at different rotations. OER LSV-curves (d), the resulting overpotentials at 10 mA/cm2 (e) and the Tafel plots (f) of NSC, Co/SA-NSC, Fe/SA-NSC, Fe,Co/DSA-NSC and IrO2 catalysts. (g) The ORR-LSV curves of the Fe,Co/DSA-NSC before and after 10,000 cycles. (i) The OER-LSV curves of the Fe,Co/DSA-NSC before and after 1000 cycles. (h) Graphical depiction of ORR/OER catalytic performance before and after stability test.

Fig. 3. (a) Oxygen-activity obtained from the ORR (E1/2) and OER (Ej=10) of NSC, Fe/SA-NSC, Co/SA-NSC, Fe,Co/DSA-NSC and Pt/C&IrO2 catalysts. (b) A graphic design of a zinc-air battery with the Fe,Co/DSA-NSC catalyst as the air-electrode. (c) Discharge-polarization and correspondent peak-power density curves of Fe,Co/DSA-NSC and Pt/C&IrO2 catalysts (d) Charging/Discharge-voltage curves. (e) Discharging-curves at 10 mA/cm2. (f) Galvanostatic discharging-profile at different current densities. (g) Charge/Discharge-cycling performance at 10 mA/cm2. (h) Bloated cycling potential gap at 10 mA/cm2.
此项研究得到了国家自然科学基金项目和深圳市面上项目的资助,深圳大学为第一完成单位。(来源:科学网)
相关论文信息:https://doi.org/10.1021/acscatal.2c05654