近日,美国斯坦福大学Brian K. Kobilka及其小组揭示RAMP2对胰岛素受体的负向异构调节作用。该项研究成果发表在2023年3月30日出版的《细胞》杂志上。
研究人员表示,受体活性修饰蛋白(RAMP)调节许多B族GPCR的活性。
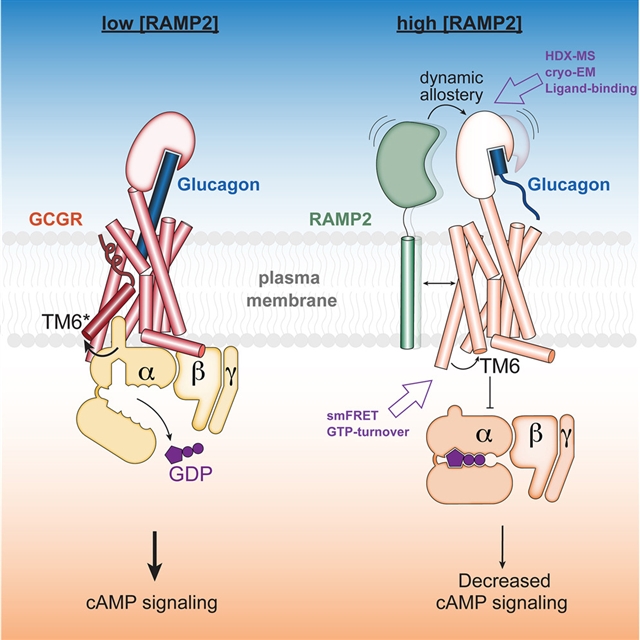
研究人员表明,RAMP2直接与负责血糖平衡的B族GPCR,即胰高血糖素受体(GCGR)相互作用,并广泛抑制受体诱导的下游信号。HDX-MS实验证明,RAMP2在受体胞外结构域(ECD)和第6跨膜螺旋中的选定位置增强了局部灵活性,而smFRET实验表明,这种ECD紊乱导致细胞内表面的活性和中间状态受到抑制。研究人员在RAMP2存在的情况下以2.9埃的分辨率测定了GCGR-Gs复合物的冷冻电镜结构。RAMP2显然没有以有序的方式与GCGR相互作用;然而,受体ECD确实在很大程度上是无序的,同时还有几个细胞内激活标志的重新排列。
这项研究表明,RAMP2通过加强ECD的构象取样,充当GCGR的负向异构调节器。
附:英文原文
Title: Negative allosteric modulation of the glucagon receptor by RAMP2
Author: Kaavya Krishna Kumar, Evan S. O’Brien, Chris H. Habrian, Naomi R. Latorraca, Haoqing Wang, Inga Tuneew, Elizabeth Montabana, Susan Marqusee, Daniel Hilger, Ehud Y. Isacoff, Jesper Mosolff Mathiesen, Brian K. Kobilka
Issue&Volume: 2023/03/30
Abstract: Receptor activity-modifying proteins (RAMPs) modulate the activity of many FamilyB GPCRs. We show that RAMP2 directly interacts with the glucagon receptor (GCGR),a Family B GPCR responsible for blood sugar homeostasis, and broadly inhibits receptor-induceddownstream signaling. HDX-MS experiments demonstrate that RAMP2 enhances local flexibilityin select locations in and near the receptor extracellular domain (ECD) and in the6th transmembrane helix, whereas smFRET experiments show that this ECD disorder resultsin the inhibition of active and intermediate states of the intracellular surface.We determined the cryo-EM structure of the GCGR-Gs complex at 2.9 resolution in the presence of RAMP2. RAMP2 apparently does not interactwith GCGR in an ordered manner; however, the receptor ECD is indeed largely disorderedalong with rearrangements of several intracellular hallmarks of activation. Our studiessuggest that RAMP2 acts as a negative allosteric modulator of GCGR by enhancing conformationalsampling of the ECD.
DOI: 10.1016/j.cell.2023.02.028
Source: https://www.cell.com/cell/fulltext/S0092-8674(23)00171-X
